Cobalt
Introduction
"Moreover, another metal is produced from cobalt. It is fluid like zinc, with a peculiar black colour, beyond that of lead and iron, possessing no brightness or metallic sparkle. It is capable of being wrought, and is malleable, but not to such and extent as to fit it for practical use. The ultimate matter of this substance has not as yet been discovered, nor its method of preparation. There is little doubt that the male and female elements are joined in its constitution, as in the case of iron and steel. They are not capable of being wrought, but remain such as they are, until Art shall discover the process for separating them."
"An imperfect metal is made from cobleta. This admits of liquefaction, and passes into a state of flux, is of a blacker colour than lead and iron, but of no brightness or metallic glitter; it barely admits of malleation, so that scarely anything can be made from it. Its ultimate matter has not yet been dicovered, nor the process of its separation. There is no doubt it is a promiscuous race from the male and female, as is the case in iron and steel, but these cannot be perfectly welded until some method of separation is discovered."
Chemical
Cobalt is not sensibly altered by exposure to air or water at ordinary temperatures, but becomes superfically oxidised at red heat. The metal dissolves rapidly at room temperature in nitric acid, slowly in hydrochloric acid and dilute sulphuric acid, evolving hydrogen.
Hydrogen is readily occulded by cobalt to an extent dependant upon a variety of factors -
1. Temperature - Cobalt slowly occludes hydrogen in the cold, but at its temperature of reduction, namely 400°C to 500°C, its occluding power is negligible. At some intermediate temperature occulusion progresses at a maximum rate. Cobalt obtained by reduction in hydrogen but cooled in nitrogen occludes an inappreciable quantity of hydrogen.
2. Length of exposure - The amount of occulded hydrogen tends towards a maximum with increasing length of exposure of the metal to that gas.
3. Physical condition of the metal - The more finely divided the metal the greater is its power of occulding hydrogen. Neuman and Streintz observed that repeated oxidation and reduction tends to diminish the power of cobalt to occlude hydrogen.
Methane is a chemical compound with the chemical formula CH₄. It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas.
Structure
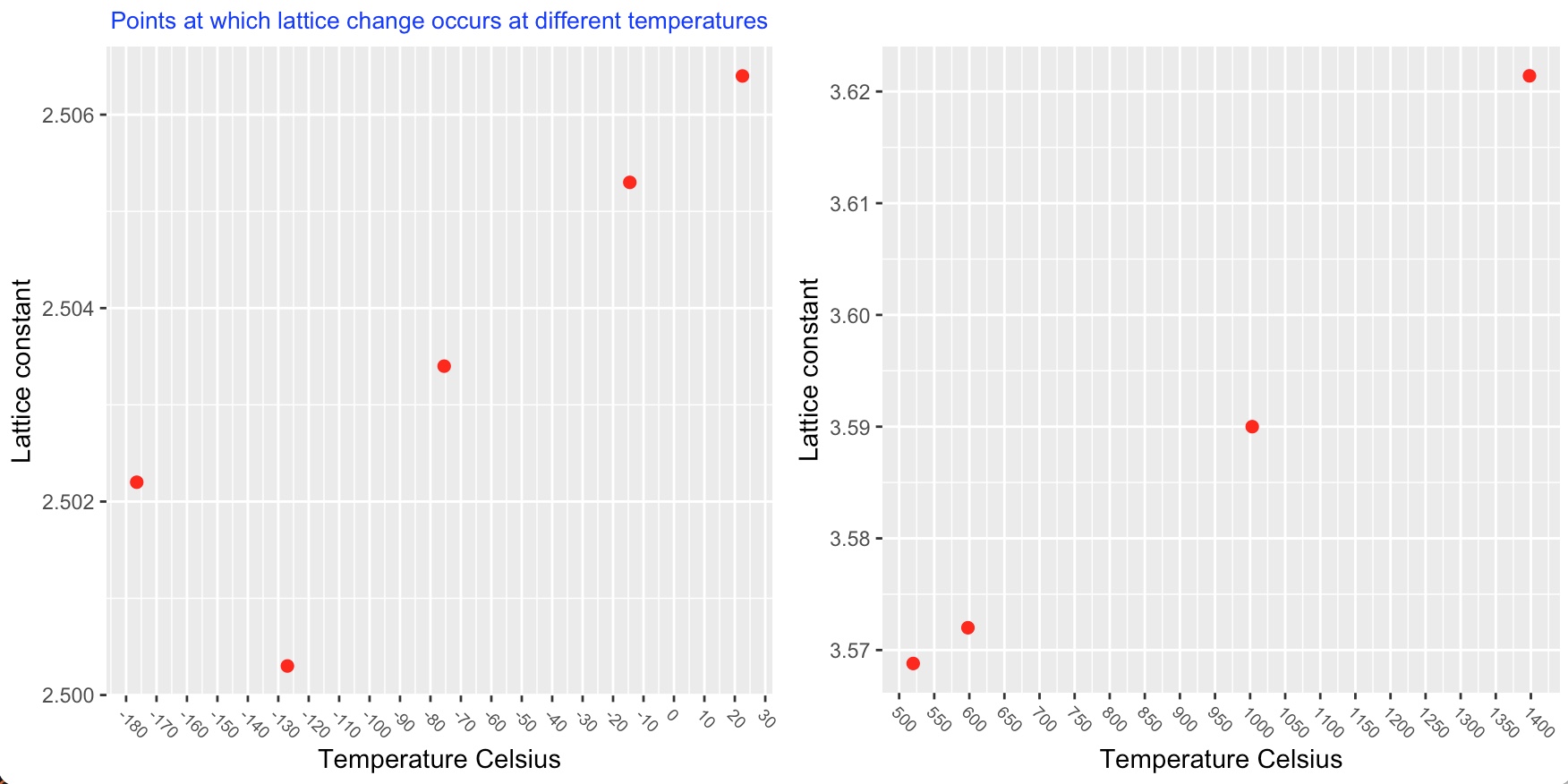
The lattice structure of cobalt changes with an increase in temperature. The stable structure of cobalt is found to be dependant upon grain size. Upto 450°C when the grain size is very small i.e powder, the structure is face centred cubic. When the grain size is larger as in a solid rod, the stable structure is close packed hexagonal. When there is a range of grain size as in fine filings a mixture of these two structures is observed.
At temperatures above 450°C the cobalt powder, filings and rod all now show a face centred cubic structure.
Several investigators believe that there is a second allotropic transformation of cobalt (f.c.c to h.c.p) at or near the Curie point, while others have presented evidence that f.c.c is stable from 450°C to at least 1350°C. There are indications that the occurence of a h.c.p phase at high temperatures is dependant on the presence of elements such as carbon or nitrogen.
The following charts show the temperatures at which a lattice structure change occurs.
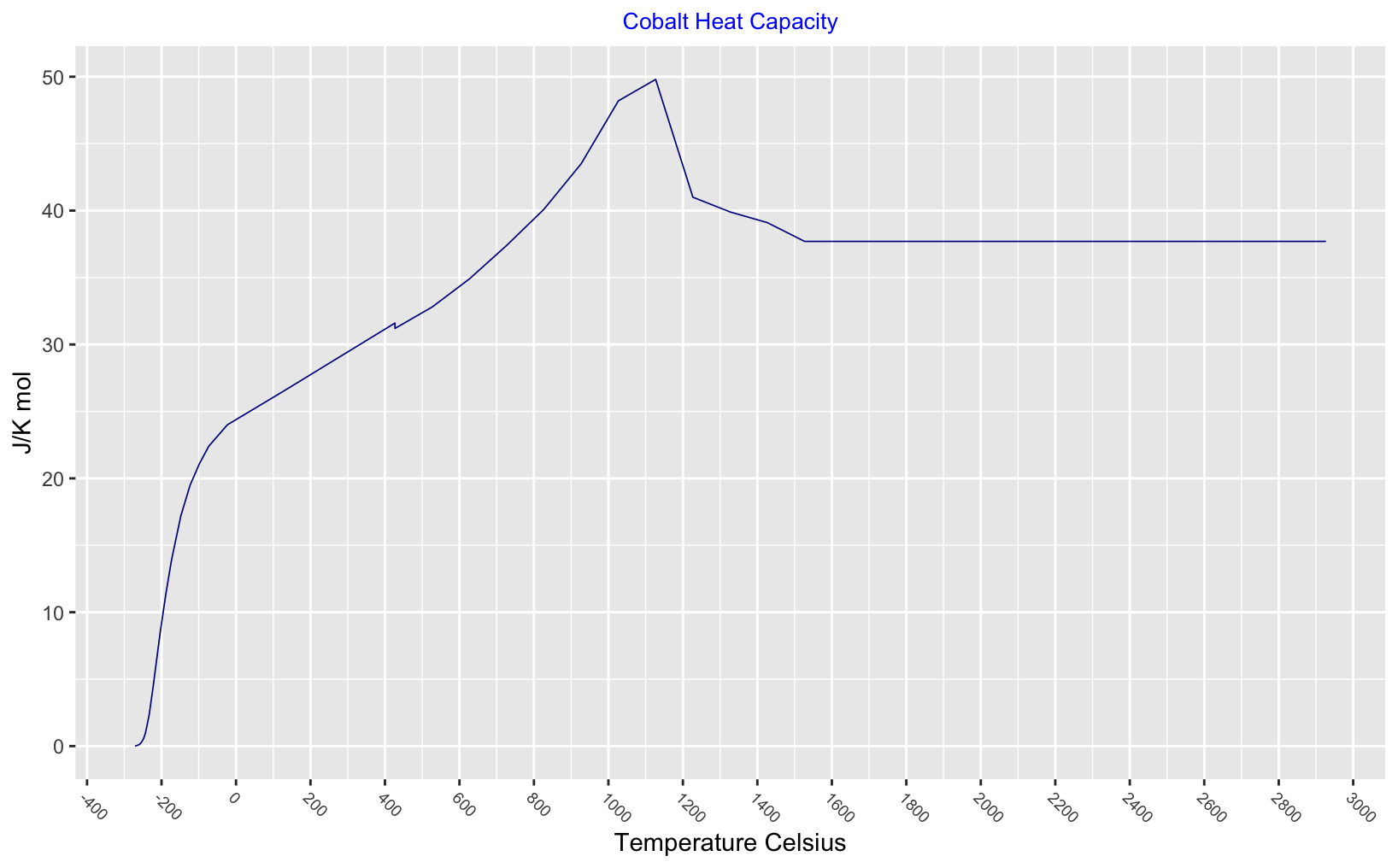
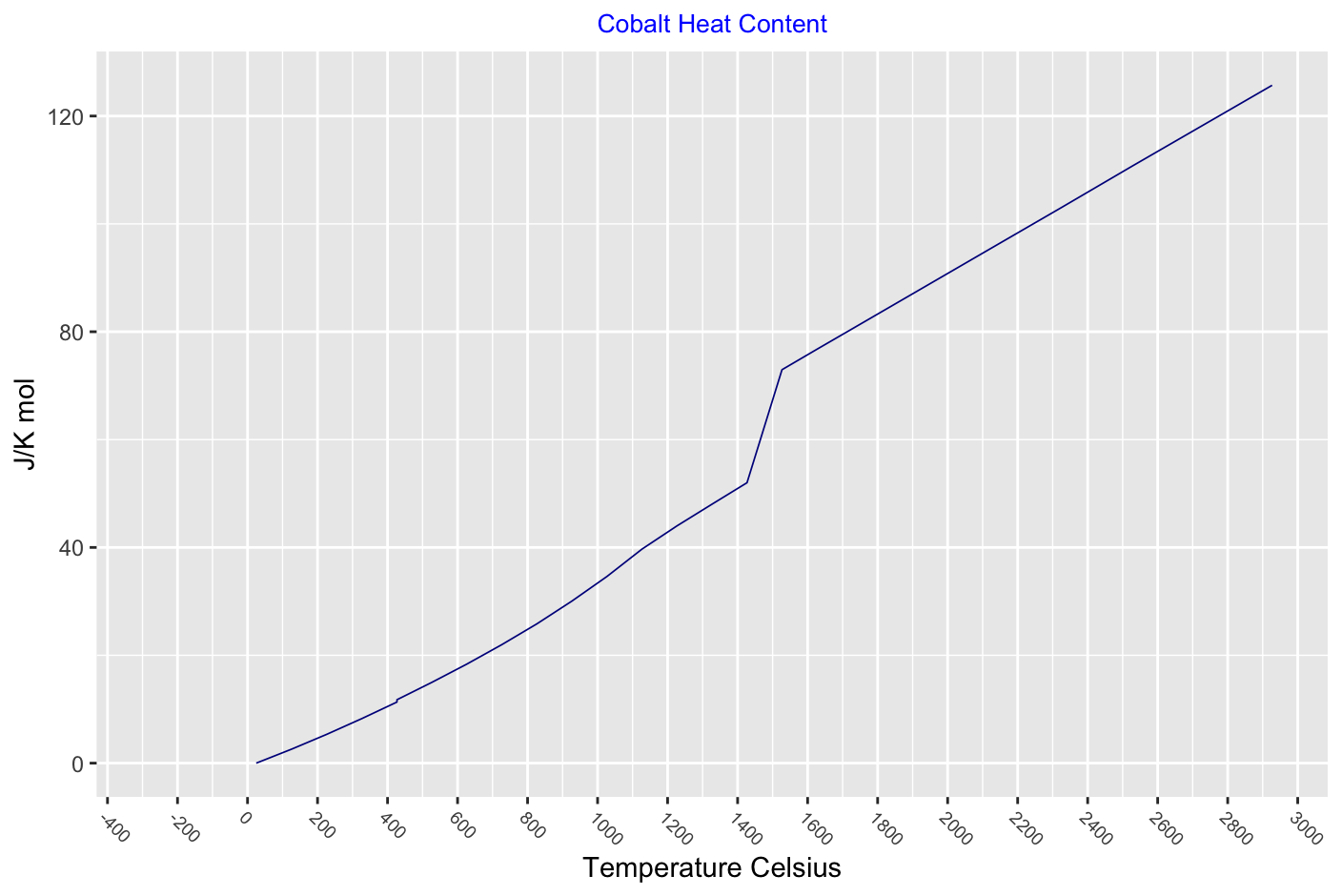
Heat
Thermodynamic functions for a solid and liquid cobalt - there is a slight drop in heat capcity at the phase change 426°C and heat capacity peaks at the Curie point.
Optical
The fundamental optical constants of a metal are the index of refraction and the extinction coefficient, which are related to the conductivity and dielectric constant.
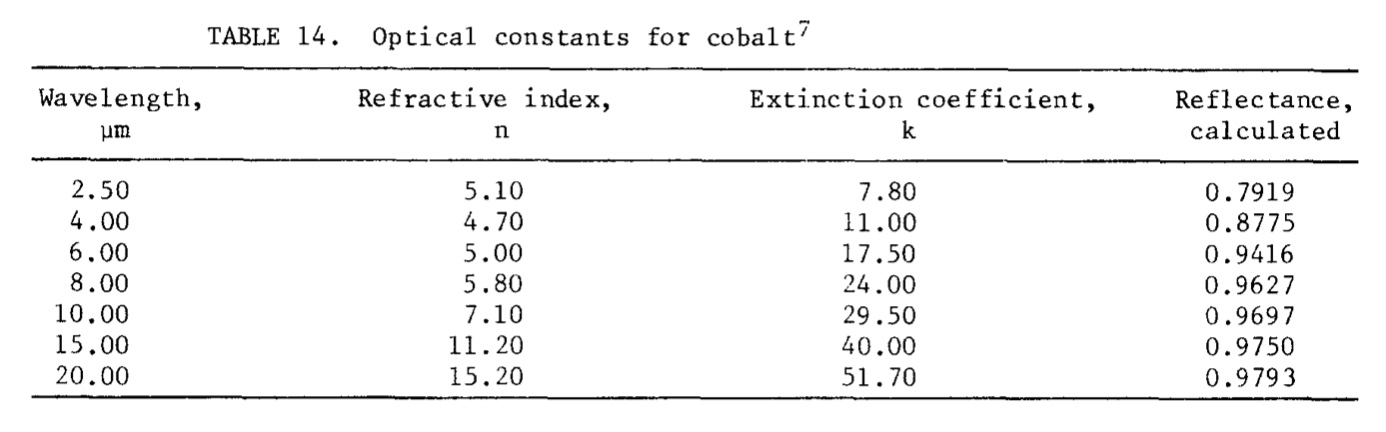
Emittance is defined as the radiant energy emitted normal to the surface divided by the corresponding emission from a black body. The spectral emittance in the infrared region as a function of temperature has been studied by Ward and Wahlin and Knop; a change was noted at or near the Curie temperature.
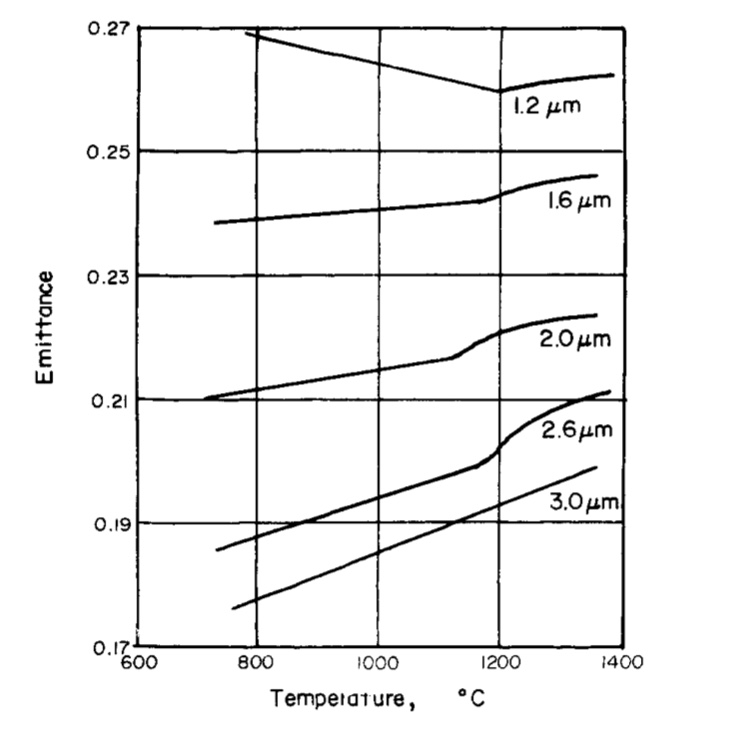
Magnetism
Cobalt is magnetic at all temperatures up to about 1150°C, at which point it becomes non-magnetic. When placed in a magnetic field a bar of cobalt increases in length, and it is interesting to note in this connection that this magnetic elongation for both cast and annealed cobalt becomes partially nil between 1100°C and 1200°C.
Magnetic anisotropy describes how a materials magnetic properties can be different depending on the direction of the magnetic field applied to it. A material that behaves the same magnetically, regardless of the direction of the field applied is isotropic. Values charted below show the hexagonal close packed structure to be highly anisotropic and after the phase change to face centred cubic cobalt becomes isotropic.
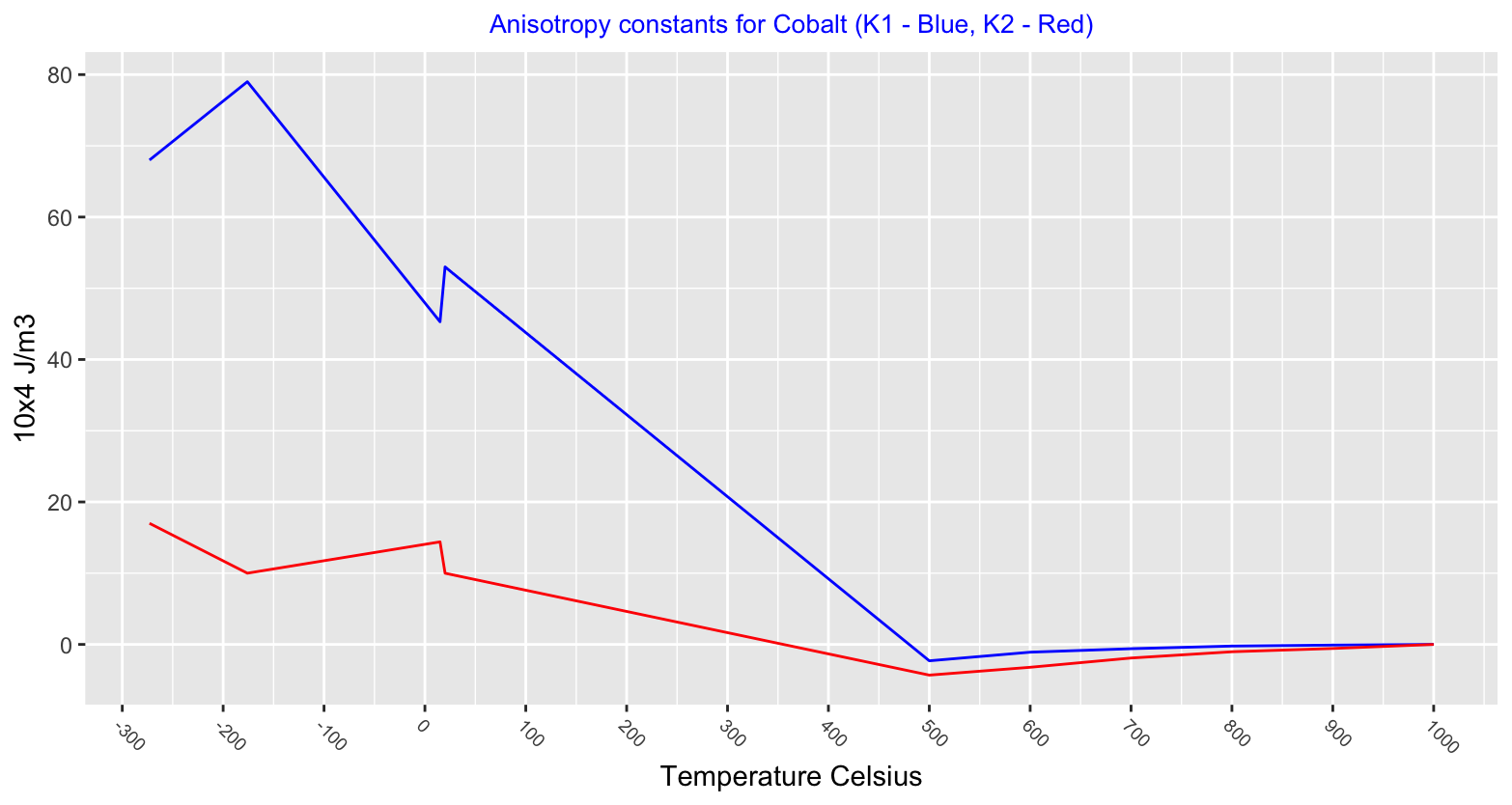
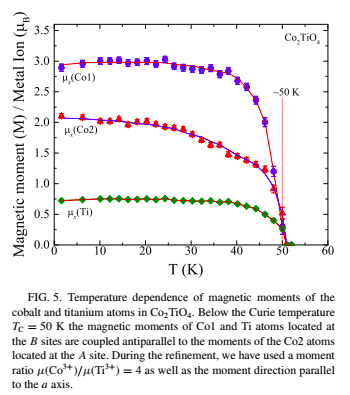
Below is chart showing a Cobalt Titanium alloy, Co2TiO4, with a Curie temperature of 50°K (-223°C), below this temperature Cobalt exhibits two magnetic states.
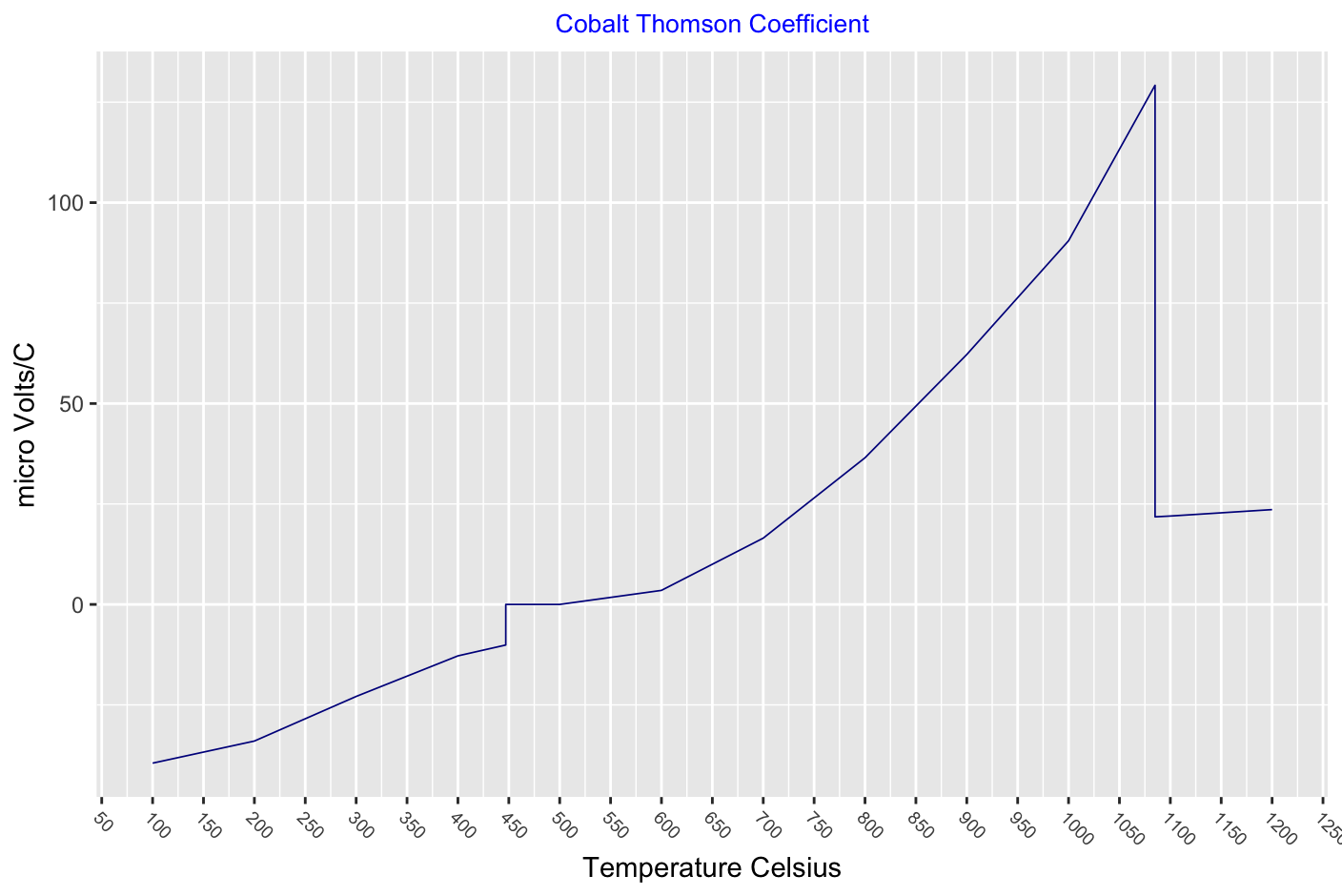
Electrical
Thomson effect, the evolution or absorption of heat when electric current passes through a circuit composed of a single material that has a temperature difference along its length. This transfer of heat is superimposed on the common production of heat associated with the electrical resistance to currents in conductors.
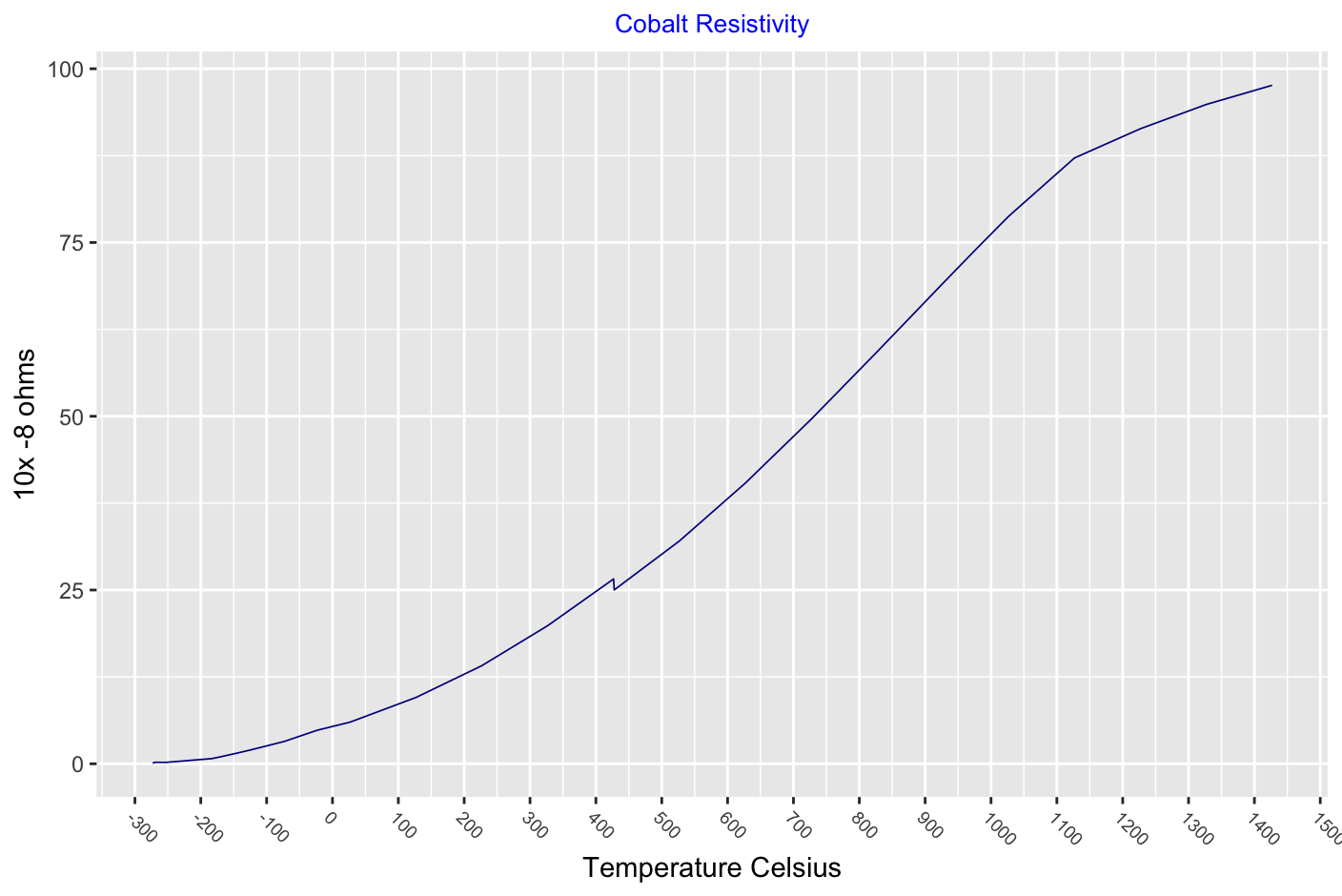
Carbon
Cobaltous Carbonate - CoCO3 - When cobalt chloride is heated to 140°C with a solution of sodium hydrogen carbonate saturated with carbon dioxide, anhydrous cobalt carbonate is produced as a bright red crystalline powder.
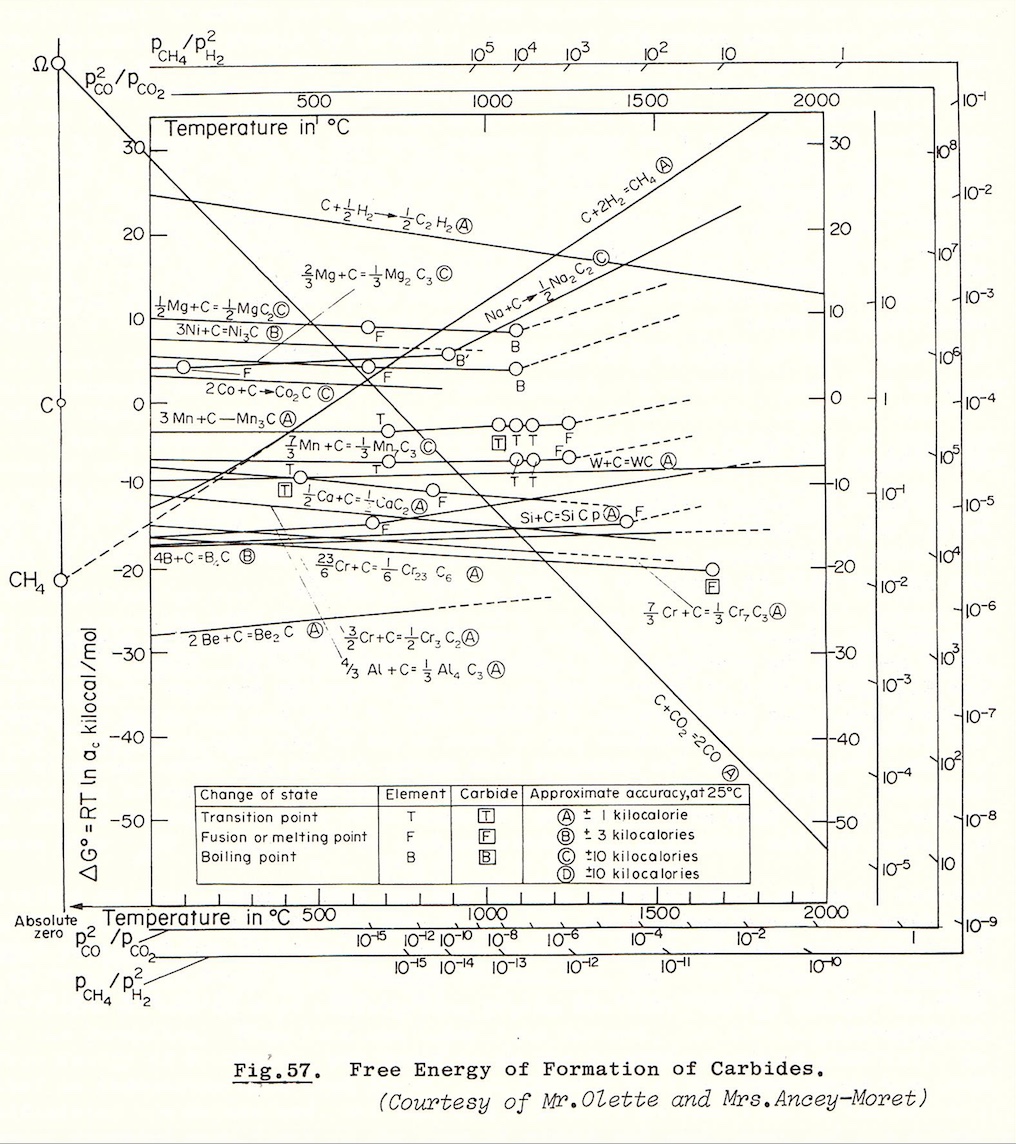
Carbon dissolves in molten cobalt at very high temperatures the carbide, Co3C, analogous to cemetite, Fe3C, appears to be formed. It decomposes so rapidly on cooling into cobalt and graphite that the carbide cannot be detected, even in quenched specimens
Pluto
The surface pressure of the atmosphere of Pluto, measured by New Horizons in 2015, is about 1 Pa (10 μbar), roughly 1/100,000 of Earth's atmospheric pressure. The temperature on the surface is 40 to 60 K (−230 to −210 °C). Near the altitude of 30 km it reaches 110 K (−163 °C), and then slowly decreases.
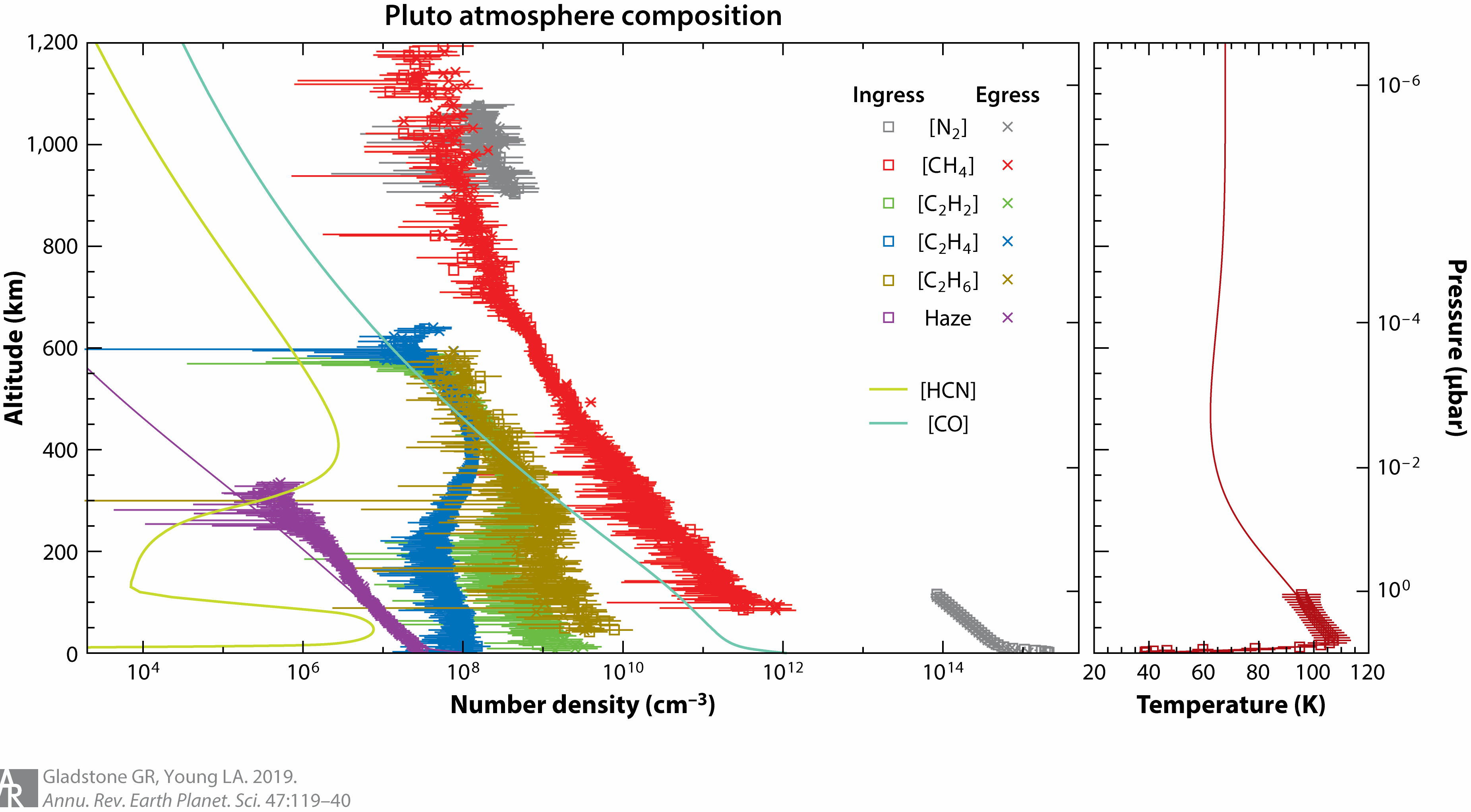
Molecular nitrogen (N2) dominates the atmosphere (at altitudes of less than 1800 kilometers or so), whereas methane (CH4), acetylene (C2H2), ethylene (C2H4), and ethane (C2H6) are abundant minor species and likely feed the production of an extensive haze that encompasses Pluto. The cold upper atmosphere shuts off the anticipated enhanced-Jeans, hydrodynamic-like escape of Pluto’s atmosphere to space.
The near-infrared reflectance spectra of Pluto and its satellites are rich with diagnostic absorption bands of ices of CH4, N2, CO, H2O, and an incompletely identified ammonia-bearing molecule.
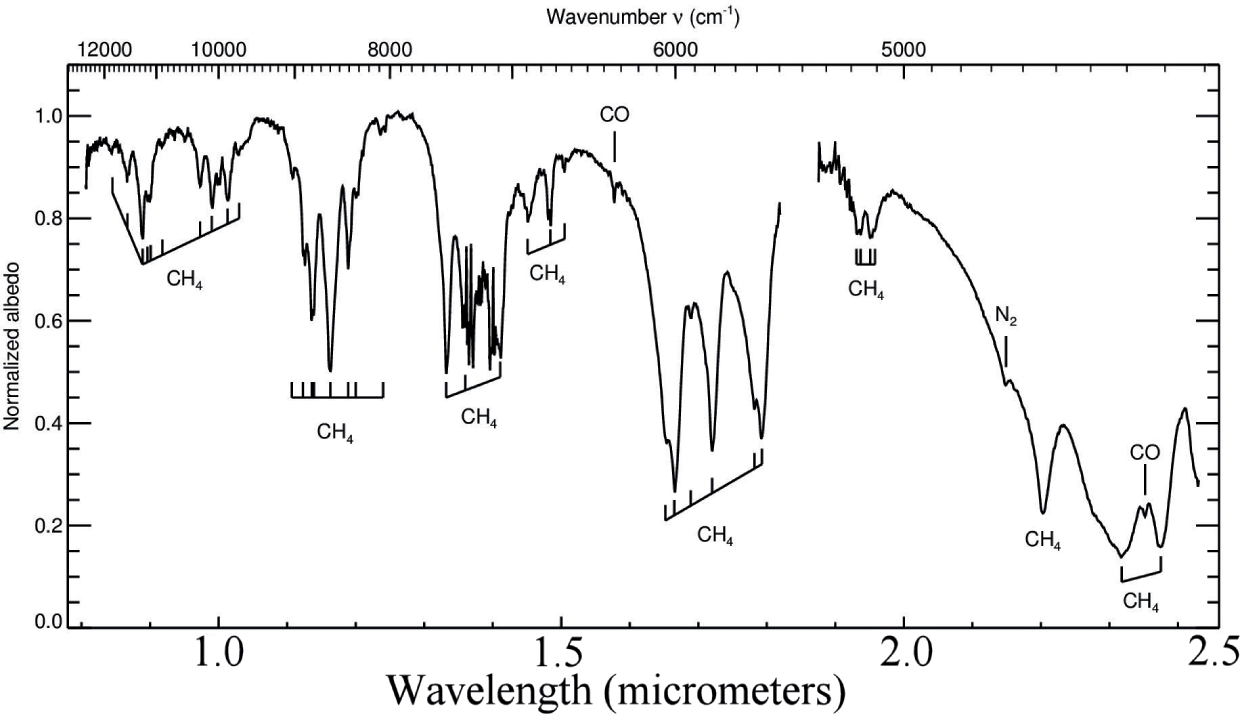
Regarding the CH4 vertical profile, we find that the data are consistent with uniform mixing in a significant fraction of the atmosphere, at least 22-27 km and probably more, and rule out situations in which methane is concentrated in near-surface cold layers. This also agrees with the predictions of Forget and Vangvichith (2013) who find that, except for a ~5 km-thick methane-depleted near-surface layer, CH4 is essentially uniformly mixed in the bulk of the atmosphere (at least from ~5 to ~40 km)
There is no evidence for an altitude-varying methane distribution. Rather, the methane mixing ratio is roughly uniform in at least the first 22-27 km of the atmosphere, and high concentrations of low-temperature methane near the surface can be ruled out. It is notable that the observed concentration of methane is 2 orders of magnitude higher than expected from Raoult's law on the basis of its concentration in surface ice and the ratio of the sublimation pressures of methane and nitrogen. Reasons of this discrepancy are unknown.
- Pluto's atmosphere composition percentages are -
- 99% Nitrogen
- 0.5% Methane
- 0.05% Carbon monoxide
Below is a chart from the New Horizons mission. It is interesting to note that some of the hydrocarbon products do not make the upper atmosphere, whereas methane, carbon monoxide and hydrogen cyanide all do.
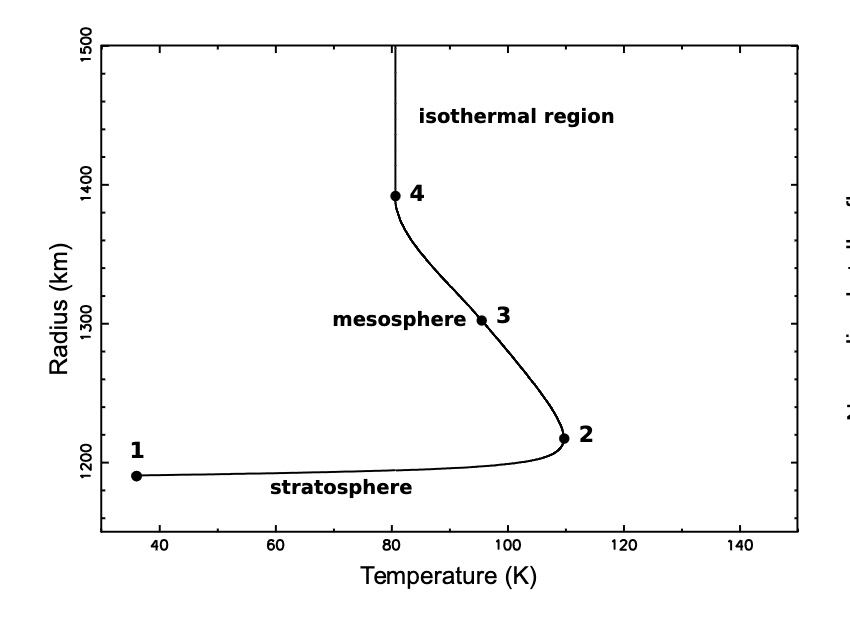
Temperature profile of Pluto's atmosphere
Planet radius 1187km - Atmosphere thickness 1100km
The chart shows a strong increase of temperature just above the surface (stratosphere), followed by a turning point where the temperature reaches a maximum (stratopause), then a region with a mild negative gradient (mesosphere), and finally an isothermal upper branch.
References
Cobalt, Nickel, and the Elements of the Platinum Group - J. Newton Friend
Cobalt, Its Occurrence, Metallurgy, Uses and Alloys - C.W. Drury
The Properties of Metallic Cobalt - W. Betteridge
Effect of grain size on the crystal structure of Cobalt - IOP Science
Neutron diffraction study of the inverse spinels Co2 TiO4 and Co2 SnO4 - 2017
Fundamentals of Metallurgical Processes - International Series on Materials Science Vol 27
New Horizons Observations of the Atmosphere of Pluto - 2019
Pluto’s atmosphere from stellar occultations in 2012 and 2013
Exploring the spatial, temporal, and vertical distribution of methane in Pluto’s atmosphere - 2014